Abstract
Manufacturing of chimeric antigen receptor (CAR) T-lymphocytes is a complex process beginning with leukapheresis of autologous lymphocytes. Tisagenlecleucel is a CAR T product licensed for treatment of patients with relapsed/refractory (r/r) diffuse large B-cell lymphoma (DLBCL), r/r B-acute lymphatic leukemia (ALL) and r/r follicular lymphoma. Production success and thus treatment outcome depends on successful collection of autologous lymphocytes.
Clonal hematopoiesis (CH) describes the outgrowth of somatically mutated hematopoietic stem cells and their progeny. The relevance of CH-related mutations in the context of CAR T-cell production is largely unknown. Here, we analyzed potential effects of CH on the manufacturing process of tisagenlecleucel.
We retrospectively analyzed leukapheresis preparations of 51 patients (13 female and 38 male; median age 61 years [y], range 20y - 80y) scheduled to undergo treatment with tisagenlecleucel. Fifty patients suffered from DLBCL and one from ALL. Thirty-five collections (68.6%) resulted in successful manufacturing, 6 (11.8%) were out of specification due to indeterminate mycoplasma tests, and 10 (19.6%) showed insufficient proliferation and expansion of CAR T-cells. All collections were analyzed using a custom, targeted deep sequencing approach covering 82 regions in 24 known CH and AML-related genes. Computational correction of sequencing errors via unique molecular identifiers enabled the reliable detection of mutations ≥0.5% variant allele frequency (VAF).
In 27 collections (53%), no CH related mutations were detected, while CH-associated mutations with VAF <2% were identified in 11 (22%), and CH with VAF ≥2% in 13 (25%). CH was present in 47% of products that resulted in successful productions, and in 33% and 40% of the collections with indeterminate mycoplasma test and insufficient proliferation, respectively (Tab. 1). Clone size did not differ between these groups with median VAFs of 1.5%, 1.5% and 1.9% (p<.92), respectively. In our population, the frequency of CH did not associate with age (p=.16), sex (p=.75), number of prior treatment lines (<3/≥3; p=.67), previous treatment with bendamustine (p=.27) or bone marrow infiltration (p=.16).
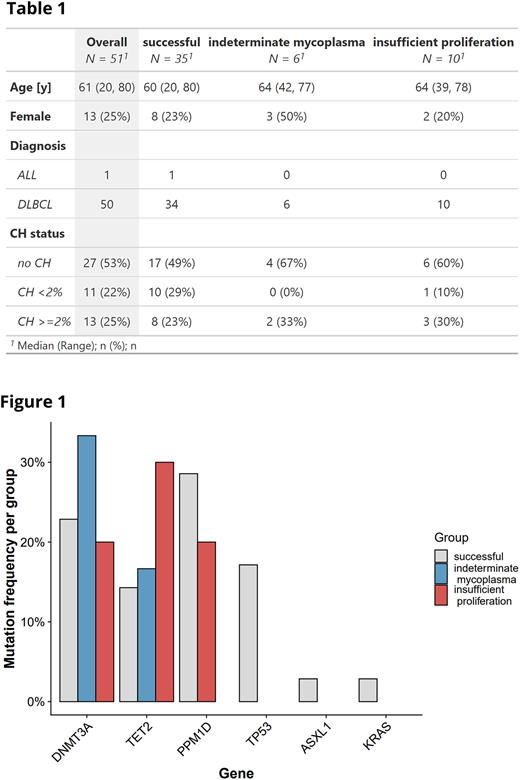
Overall, the most commonly mutated genes were DNMT3A (12/51, 24%), PPM1D (12/51, 24%) and TET2 (9/51, 18%). CH mutation spectrum was not significantly associated with manufacturing success (Fig. 1). The prevalence of mutations in TP53 and PPM1D did not differ according to number of prior treatment lines (<3/≥3; p=1) or previous treatment with bendamustine (p=0.18).
Median collected CD3+ yields were similar in apheresis products without and with CH (4.66 [range 0.92 - 19.23] x109 vs. 5.17 [range 0.97 - 31.98] x109; p=.94). Collections without and with CH also did not differ regarding CD3+CD4+ yields (2.06 [range 0.30 - 10.27] x109 vs. 2.24 [range 0.26 - 6.29] x109; p=.86) or CD3+CD8+ yields (2.62 [range 0.24 - 16.41] x109 vs. 2.76 [range 0.38 - 26.68] x109; p=.94).
CH-related mutations are common in patients undergoing treatment with CAR T-cells and are not associated to factors such as age, sex, previous treatment with bendamustine, bone marrow infiltration or previous treatment lines. The spectrum of CH driver mutations in this patient cohort differs from age-associated CH, with a notably higher frequency of PPM1D mutations. However, the presence of CH did not affect the manufacturing success of tisagenlecleucel CAR T-cells. Further analyses are required to determine the impact of CH on long-term outcomes after CAR T therapy, for example with regard to secondary myeloid neoplasms.
Disclosures
Penack:SOBI: Membership on an entity's Board of Directors or advisory committees; Incyte: Research Funding; Priothera: Membership on an entity's Board of Directors or advisory committees, Research Funding; Omeros: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Jazz: Membership on an entity's Board of Directors or advisory committees; Equillium Bio: Membership on an entity's Board of Directors or advisory committees; Therakos: Honoraria; Pfizer: Honoraria; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; MSD: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Shionogi: Membership on an entity's Board of Directors or advisory committees. Janz:Novartis: Honoraria. Bullinger:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bayer Oncology: Research Funding; Jazz Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Honoraria; Celgene/BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Keller:Novartis: Honoraria. Schwind:Novartis: Honoraria. Merz:Janssen: Honoraria; BMS Celgene: Honoraria. Jentzsch:Novartis: Honoraria. Franke:Jazz Pharmaceuticals: Honoraria; Pfizer: Honoraria; Novartis: Honoraria; Takeda: Other: Travel support; Gilead: Other: Travel support; Incyte: Honoraria; BMS: Honoraria. Herling:Novartis: Honoraria. Platzbecker:Jazz: Honoraria; Janssen: Honoraria; Silence Therapeutics: Honoraria; Takeda: Honoraria; BMS/Celgene: Honoraria; Geron: Honoraria; Abbvie: Honoraria; Novartis: Honoraria. Metzeler:Novartis: Consultancy; Abbvie: Consultancy; Celgene: Consultancy; Curis Inc: Research Funding; Jazz: Consultancy; Pfizer: Consultancy. Vucinic:Novartis, Gilead Kite, Takeda, MSD, BMS Celgene, Abbvie, Amgen: Honoraria; MSD, BMS Celgene, Novartis, Gilead Kite, Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sobi, BMS Celgene: Other: travel, accommodations, expenses.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal